We are fascinated by the fundamental mechanisms through which the heart adapts in health and disease. This process, called cardiac remodeling, represents the heart’s response to physiological or pathological stress. Depending on the context, cardiac remodeling can drive an athlete towards Olympic gold, or prevent a heart failure patient from getting out of her favorite chair. Our work at the bench often begins with genomics, studying athletes’ and patients’ DNA or RNA. We start by thinking globally —for example by defining graph relationships among genes in the failing heart. But to eventually effect change in our patients’ diseases, we have to act locally: we must establish causality and understand pathways we might want to target for therapy.
To accomplish this, we grow induced pluripotent stem cells and differentiate them into cardiomyocytes (Figure 1) , skeletal myocytes, or neurons. We use genome engineering technologies including CRISPR-Cas9-based systems to manipulate gene sequences or their expression. These perturbations allow us to study the effects of genetic variation on biological processes like cardiac hypertrophy, cardiac failure, and arrhythmia.
In fact, we increasingly study these processes at single cell resolution: We can exert fine control over load in single cardiomyocytes by using stretch-based systems to measure force, calcium, or voltage from individual myocytes. This is achieved through a technique performed at only a small number of laboratories world-wide (Figure 2).
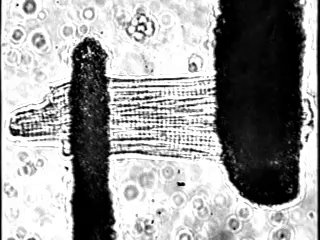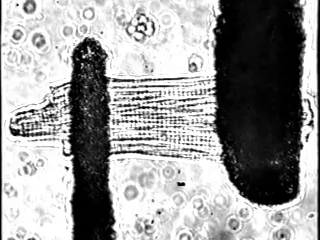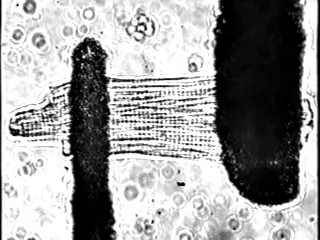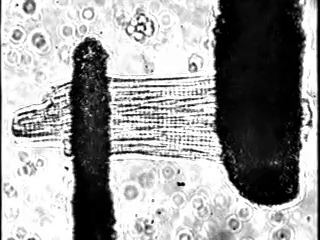
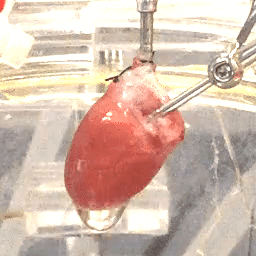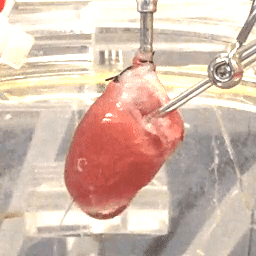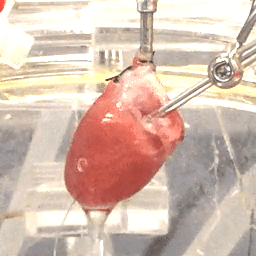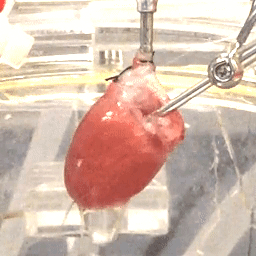
We also study the heart at the whole organ level using our in-house Langendorff system (Figure 3). This system allows us to explore the effects of potential new therapeutics or specific genomic variants on calcium handling and ex vivo hemodynamics in the entire organ.
Finally, we carry out studies in vivo measuring pressure-volume hemodynamics using a catheter or exercise testing in a variety of model organisms.